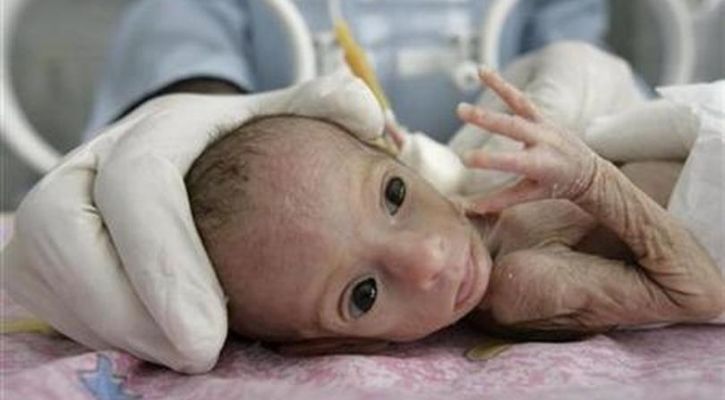
Although human trials for artificial wombs intended to save premature babies have not yet been conducted, they have exhibited significant potential in animal experiments.
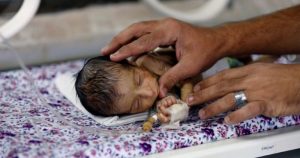
Prematurely born infants frequently confront a lifetime of health hurdles, encompassing issues such as respiratory complications, developmental delays, and the possibility of developing cerebral palsy. (Photo: Google)
Gathering to discuss the potential of artificial wombs intended to save premature babies, a panel of independent advisers to the US Food and Drug Administration (FDA) aims to enhance survival rates and mitigate long-term health concerns for extremely premature infants.
According to an article published by CNN, a panel of independent advisers to the US Food and Drug Administration (FDA) is convening to explore the concept of artificial wombs intended to save premature babies and their potential to save extremely premature babies, ultimately improving their chances of survival and reducing long-term health issues. While artificial wombs intended to save premature babies have not yet been tested in humans, they have shown promise in animal experiments.
Premature birth is a pressing issue in the US, with rates on the rise, disproportionately affecting African Americans. Babies born prematurely often face a lifetime of health challenges, including respiratory problems, developmental delays, and cerebral palsy.
Artificial wombs intended to save premature babies are not meant to replace a natural pregnancy but could be used for infants born before 28 weeks, considered extremely premature. These devices would provide oxygen, nutrients, and hormones critical for the final stages of lung and brain development.
READ ALSO: Congress Debates Emergency Food Assistance Program Of Further Cuts
Currently, premature infants require care in neonatal intensive care units (NICUs), which carry infection risks, and the use of ventilators can harm fragile lungs.
According to an article published by Albany Herald, before human trials of artificial wombs intended to save premature babies could begin, scientists must demonstrate that artificial wombs intended to save premature babies can support growth, reduce mortality rates, and minimize health issues when compared to traditional NICU care. The FDA’s Pediatric Advisory Committee will determine the necessary data, regulations, and ethical considerations for such trials.
Researchers have conducted successful animal experiments with artificial wombs intended to save premature babies, showing positive results in lung and brain development. However, challenges, including blood circulation and brain injury, have also been encountered.
The FDA’s decision on artificial wombs intended to save premature babies will be closely watched, as it could revolutionize care for premature infants and raise important ethical and regulatory questions.
While the panel’s discussions will influence the FDA’s approach, the agency retains the final say in its decisions. The first day of hearings is open to the public, but the second is closed due to proprietary information concerns.
READ ALSO: Amidst Challenges In Real Estate Market, Racial Disparities And Discrimination Loom As Shadows, NAR Reports